AGE 6+
FOR MILD TO MODERATE
ECZEMA (ATOPIC DERMATITIS)
RESULTS
Rapid and reliable relief in a steroid-free cream
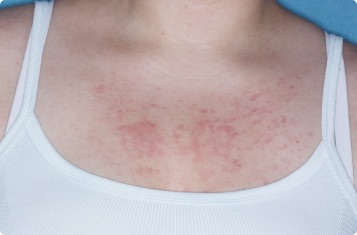
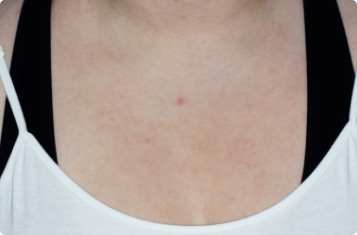
Symptoms illustrated.
Not an actual patient.
ZORYVE was evaluated as a once-daily, steroid-free, stand-alone treatment over the course of 4 weeks in 2 clinical trials of 1,337 people aged 6 and older with mild to moderate eczema. 884 people used ZORYVE daily for 4 weeks. 453 people used the same cream without the active ingredient (the inactive cream) daily for 4 weeks.
Clear or Almost Clear skin*
31% of people using ZORYVE had Clear or Almost Clear skin* in 4 weeks vs 14% of people using inactive cream
*People who had Clear or Almost Clear skin and a 2-point improvement on a 5-point severity scale. 0 = Clear, 1 = Almost Clear, 2 = Mild, 3 = Moderate, 4 = Severe.
Fast itch relief
32% of 542 people using ZORYVE had significant itch relief in 4 weeks vs 17% of 271 people using inactive cream
Symptoms illustrated. Not an actual patient.
People using ZORYVE felt greater itch relief 24 hours after first application compared to people using inactive cream
Most common side effects
These are not all the possible side effects of ZORYVE cream. Call your doctor for medical advice about side effects.
Low rates of stinging or burning (1.6%) were reported after first application of ZORYVE vs 2.0% with inactive cream
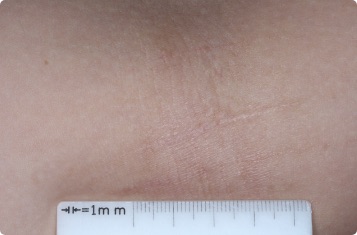
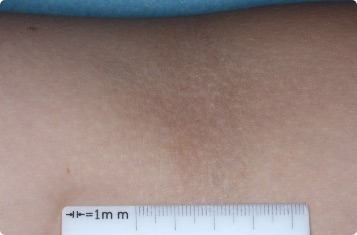
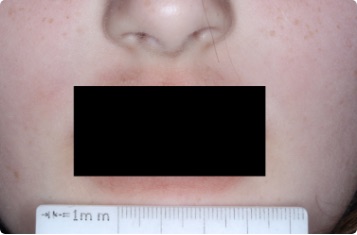
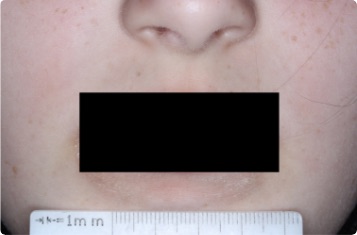
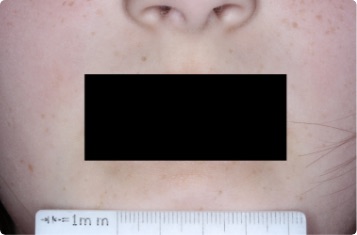
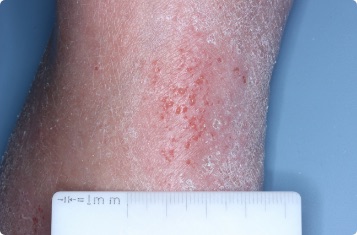
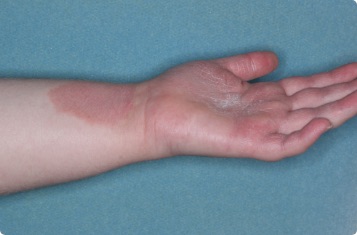
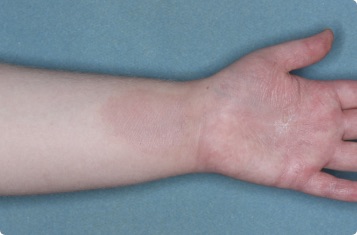
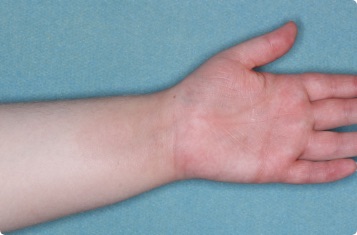
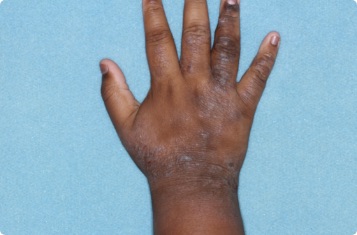
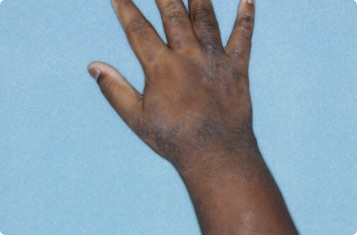
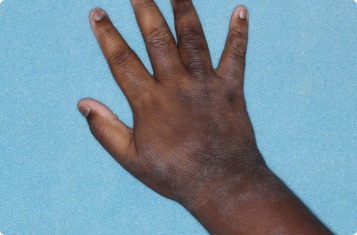
Results you can see and feel anywhere on the skin
Actual clinical trial patients.
Inside Elbow
12-YEAR-OLD
Face
9-YEAR-OLD
Ankle
65-YEAR-OLD
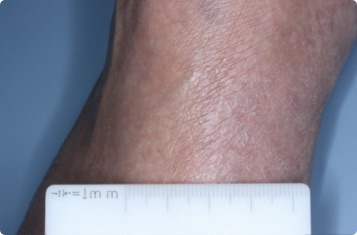
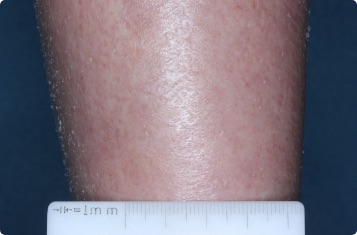
Hand & Inside Wrist
24-YEAR-OLD
Hand
11-YEAR-OLD
Chest
17-YEAR-OLD